Gastroesophageal Resuscitative Occlusion of the Aorta (GROA)
Value Proposition
Uncontrollable hemorrhage is a major cause of preventable death and the #1 cause of combat deaths for U.S. service members. GROA is a minimally invasive device that can be used to rapidly stabilize a patient by controlling severe non-compressible abdominal hemorrhage at the point of impact. It is also easily implemented in austere environments, such as battlefields or limited resource settings.
Competitive Advantage
Easy to Implement
Minimally Invasive
Works Alongside Secondary Treatments
Unique Features
Easy to Implement
Minimally invasive
Works alongside secondary treatments
Rapidly stabilizes a patient by controlling severe non-compressible abdominal hemorrhage at the point of impact.
Allows partial to full mechanical occlusion
Licensing Information
Precision Trauma in 2019
Principal Investigators
Kevin Ward, MD
Hakam Tiba, MD, MS
Jeff Plott, PhD
Intellectual Property
Invention Disclosure # 2019-106
Patent Application Submitted
Solution Sheet
Download Solution Sheet (PDF)
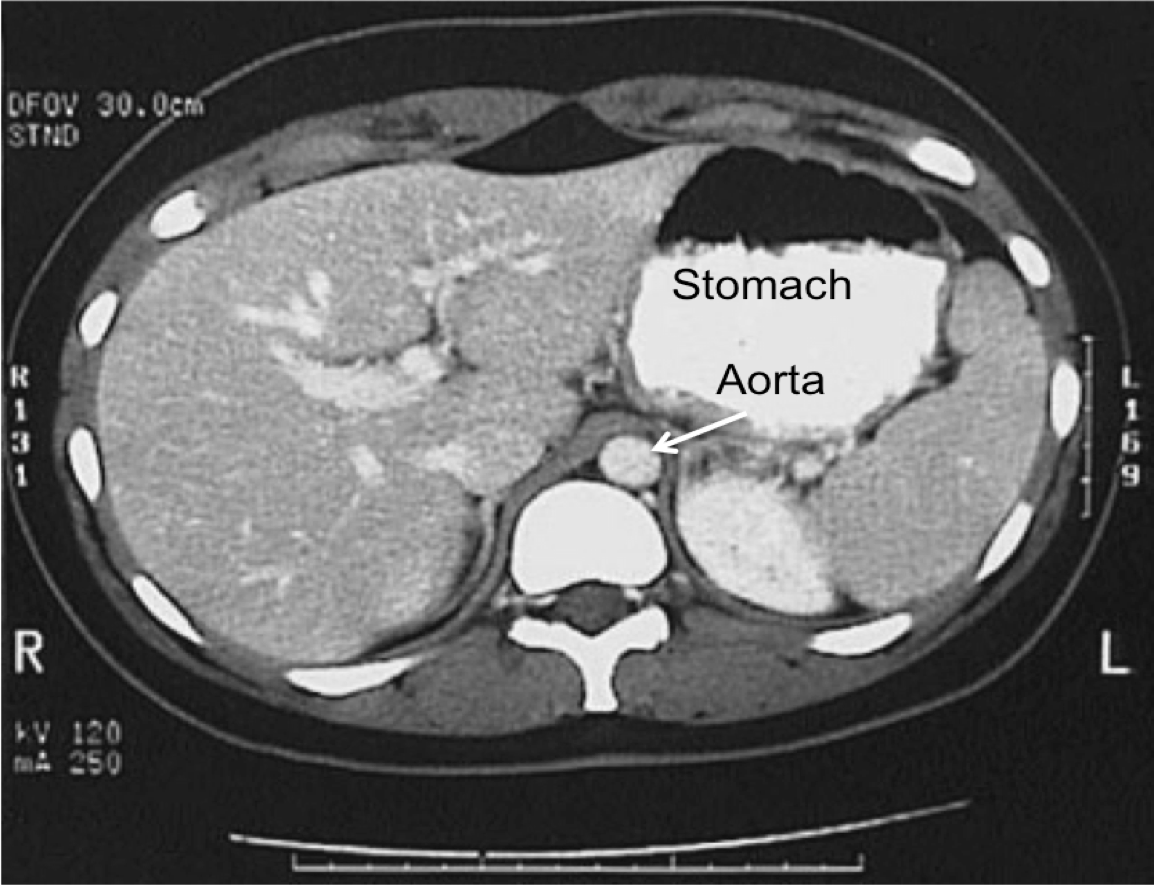
Similar to the successful catheter known as REBOA (Resuscitative Endovascular Balloon Occlusion of the Aorta) which is an invasive device frequently used in hospital settings, The GROA device is capable of achieving high zone II full aortic occlusion and may be able to serve as an effective method of aortic impingement. As opposed to needing a catheter. GROA effectively controls non-compressible abdominal hemorrhage through a minimally-invasive, and easy-to-implement device.
GROA is well-suited for saving lives in limited resource settings, during EMS treatment and transportation, and on the battlefield.
Contact Precision Trauma for more information about investment and partnership opportunities.
Funding History
This work is supported by the Office of the Assistant Secretary of Defense for Health Affairs through the JPC-6 Combat Casualty Care / Defense Medical Research and Development Program under an assistance agreement from the U.S. Army Medical Research Activity, Award No. W81XWH-18-1-0033.
Completed Milestones:
Data capture
Model testing and training
Retrospective Validation at Michigan Medicine and (externally) at Hurley Hospital
Live Prospective Validation at Michigan Medicine
MVP Version 1.0 software release: Adult and Pediatric models